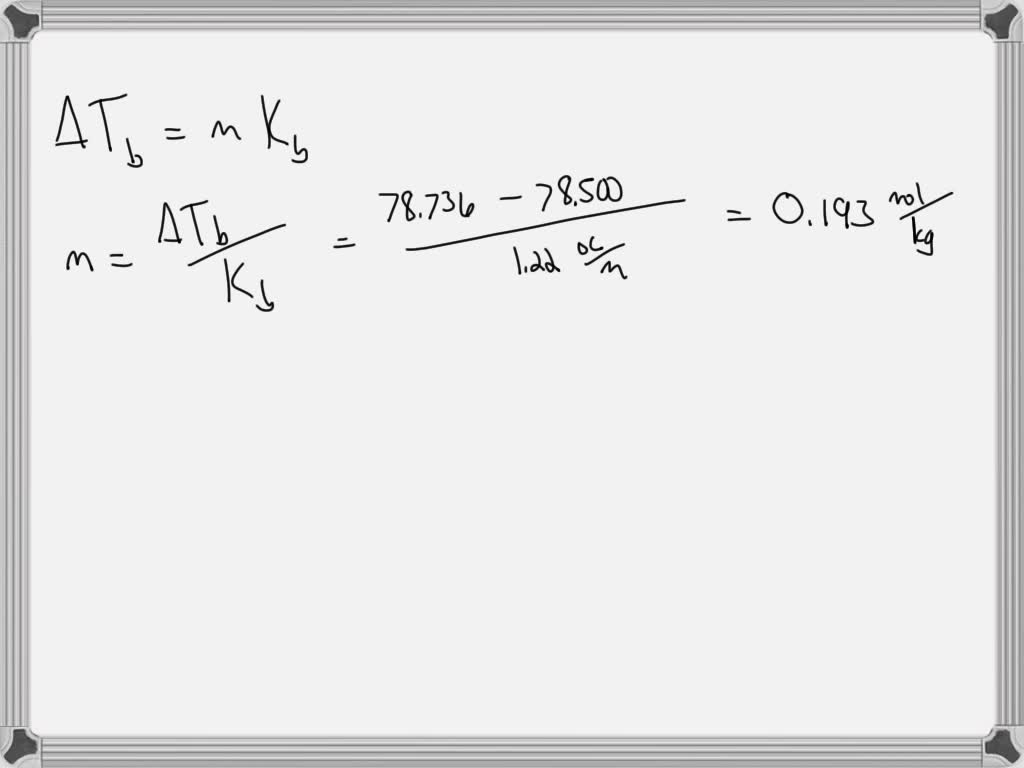Why Does Ethanol Have A High Boiling Point . Explain why alcohols and ethers of. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web pure ethanol is a colourless flammable liquid (boiling point 78.5 °c [173.3 °f]) with an agreeable ethereal odour and a burning. Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web because boiling point of different materials depend on the intermolecular forces present between the atoms.
from www.numerade.com
Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web pure ethanol is a colourless flammable liquid (boiling point 78.5 °c [173.3 °f]) with an agreeable ethereal odour and a burning. Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with. Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Explain why alcohols and ethers of.
SOLVED The boiling point of ethanol, CH3CH2OH, is 78.500 °C at 1
Why Does Ethanol Have A High Boiling Point Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Explain why alcohols and ethers of. Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web pure ethanol is a colourless flammable liquid (boiling point 78.5 °c [173.3 °f]) with an agreeable ethereal odour and a burning. Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with.
From guides.hostos.cuny.edu
Chapter 3 Solids and Liquids CHE 110 Introduction to Chemistry Why Does Ethanol Have A High Boiling Point Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Explain why alcohols and ethers of. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web explain why the boiling points of alcohols are higher than those of ethers. Why Does Ethanol Have A High Boiling Point.
From www.teachoo.com
[Chemistry] Differentiate between Ethanol and Ethanoic acid Class 10 Why Does Ethanol Have A High Boiling Point Web pure ethanol is a colourless flammable liquid (boiling point 78.5 °c [173.3 °f]) with an agreeable ethereal odour and a burning. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar. Why Does Ethanol Have A High Boiling Point.
From askfilo.com
(a) Why do acids have higher boiling points than alcohol? Filo Why Does Ethanol Have A High Boiling Point Web pure ethanol is a colourless flammable liquid (boiling point 78.5 °c [173.3 °f]) with an agreeable ethereal odour and a burning. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Web this. Why Does Ethanol Have A High Boiling Point.
From www.reddit.com
How long does distilling take? r/firewater Why Does Ethanol Have A High Boiling Point Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with.. Why Does Ethanol Have A High Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Why Does Ethanol Have A High Boiling Point Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web pure ethanol is a colourless flammable liquid (boiling point 78.5 °c [173.3 °f]) with an agreeable ethereal odour and a burning. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar. Why Does Ethanol Have A High Boiling Point.
From www.chemistrystudent.com
Alcohols (ALevel) ChemistryStudent Why Does Ethanol Have A High Boiling Point Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with.. Why Does Ethanol Have A High Boiling Point.
From www.numerade.com
SOLVED The boiling point of ethanol, CH3CH2OH, is 78.500 °C at 1 Why Does Ethanol Have A High Boiling Point Web pure ethanol is a colourless flammable liquid (boiling point 78.5 °c [173.3 °f]) with an agreeable ethereal odour and a burning. Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with. Web explain why the boiling points of alcohols are higher than those of ethers. Why Does Ethanol Have A High Boiling Point.
From mavink.com
Organic Chemistry Boiling Point Chart Why Does Ethanol Have A High Boiling Point Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with. Explain why alcohols and ethers of. Web the boiling point of ethanol can be affected by. Why Does Ethanol Have A High Boiling Point.
From www.youtube.com
Why does ethanol have a higher boiling point than ethanal? YouTube Why Does Ethanol Have A High Boiling Point Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web this table shows. Why Does Ethanol Have A High Boiling Point.
From www.numerade.com
SOLVED Model 3 Boiling Points of Alcohols Alcohol Number of Carbons Why Does Ethanol Have A High Boiling Point Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with. Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web because boiling point of different materials depend on the intermolecular forces present between the atoms.. Why Does Ethanol Have A High Boiling Point.
From www.coursehero.com
[Solved] The boiling points of ethanol and dimethyl ether are provided Why Does Ethanol Have A High Boiling Point Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with.. Why Does Ethanol Have A High Boiling Point.
From www.chegg.com
Solved The boiling point of ethanol, CH3CH2OH, is 78.50°C at Why Does Ethanol Have A High Boiling Point Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses.. Why Does Ethanol Have A High Boiling Point.
From chemizi.blogspot.com
Why boiling point of alcohol is higher than ether and alkane Why Does Ethanol Have A High Boiling Point Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Explain why alcohols and ethers of. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar. Why Does Ethanol Have A High Boiling Point.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Why Does Ethanol Have A High Boiling Point Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Explain why alcohols and. Why Does Ethanol Have A High Boiling Point.
From www.youtube.com
Boiling Point for C2H5OH (Ethanol or Ethyl Alcohol) YouTube Why Does Ethanol Have A High Boiling Point Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Explain why alcohols and ethers of. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar. Why Does Ethanol Have A High Boiling Point.
From wisc.pb.unizin.org
M10Q2 Melting and Boiling Point Comparisons Chem 103/104 Resource Book Why Does Ethanol Have A High Boiling Point Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes. Why Does Ethanol Have A High Boiling Point.
From www.coursehero.com
[Solved] Why does ethanal have a greater boiling point than propane Why Does Ethanol Have A High Boiling Point Web the boiling point of ethanol can be affected by the presence of impurities or other substances in the mixture. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses. Explain why alcohols and. Why Does Ethanol Have A High Boiling Point.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Why Does Ethanol Have A High Boiling Point Web this table shows that alcohols (in red) have higher boiling points and greater solubility in h 2 o than haloalkanes and alkanes with. Web because boiling point of different materials depend on the intermolecular forces present between the atoms. Web explain why the boiling points of alcohols are higher than those of ethers and alkanes of similar molar masses.. Why Does Ethanol Have A High Boiling Point.